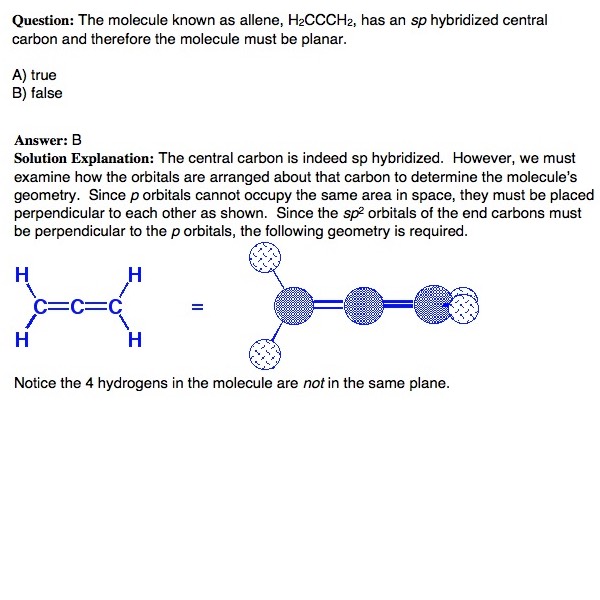
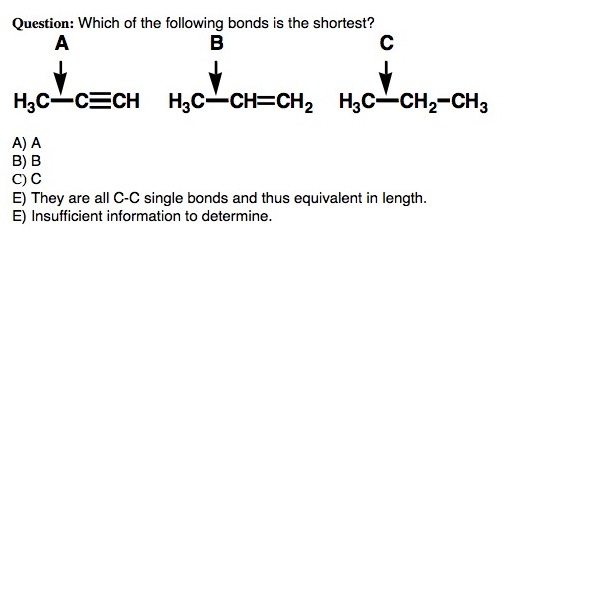
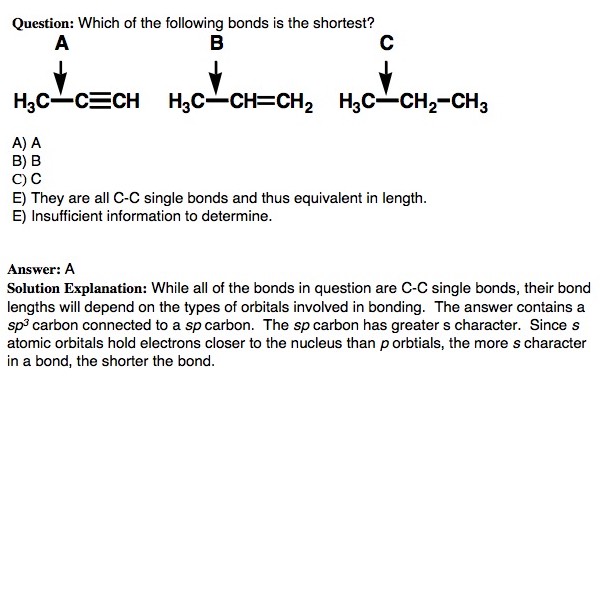
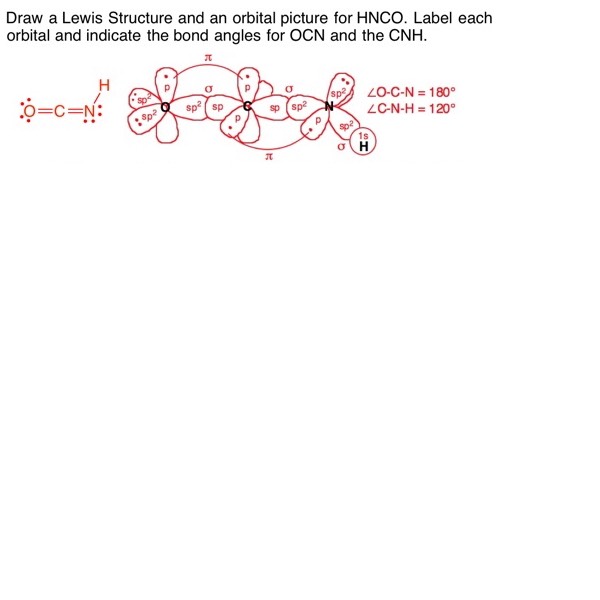
This tutorial discusses the concept of hybridization and explains how hybridized orbitals are the building blocks of organic chemistry. Using hybridization, VSEPR, and bonding theory, the tutorial shows how the shapes of molecules can easily be predicted. (Runtime = 29:18)
Student Learning Outcomes
After viewing this tutorial and practicing problems from this website and your textbook, you should be able to:
• Determine the hybridization state of every atom in an organic compound.
• Visualize and draw the geometry about an atom or group of atoms in an organic compound.
• Draw the orbital picture for any molecule or section of a molecule.
Practice Problems
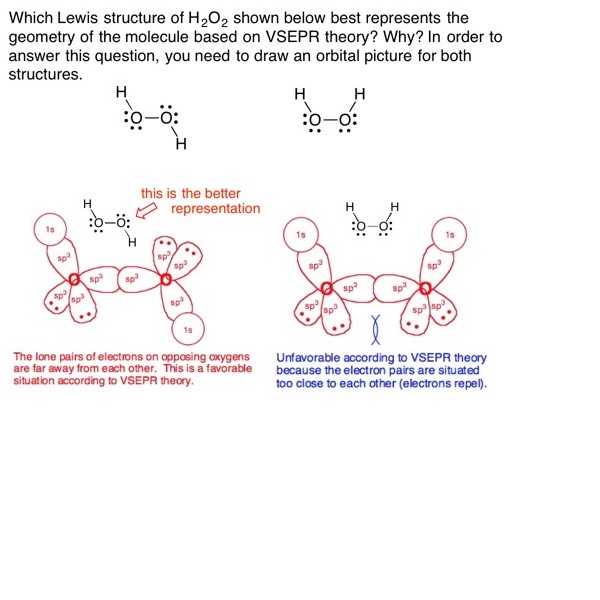
Here are some problems to get you started. You should first try each on a piece of paper. Then, place your cursor over the graphic and click on right arrow that appears to see the answer. The right and left arrows allow you to move back and forth between problems. Please note that these are not the only types of problems/questions that exist on this topic. Refer to your textbook and any problems your instructor provides you for more practice with this subject.